It has been a good while since healthcare has embarked on an empowering digital journey. Powered by consumer behavior and steady technological advancements – and despite regulatory lapses and the inherent challenges and concerns, – software continues to revolutionize and optimize health care. Even though the term SaMD may not sound familiar, this type of software is part of our daily routine more than we think. If you have heard – or even used – of verified mobile apps that recommend insulin dosages depending on the patient’s food logs, fertility apps, or real-time blood pressure reading, then you already know some of the functions of SaMD. Defined as standalone software that is a medical product ITSELF, the SaMD market is expected to grow at a CAGR of 11.0% between 2021 and 2027, reaching $8.2 billion by 2027. Compounded with groundbreaking technologies such as AR/VR, AI/ML, or IoT, SaMD has already become a game-changer, playing a crucial role in disease prevention, diagnosis, monitoring, and treatment. Allow us to unravel the hidden potential of SaMD!
What is SaMD?
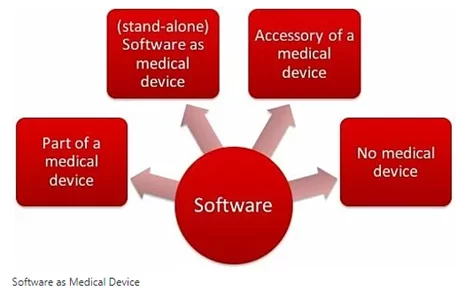
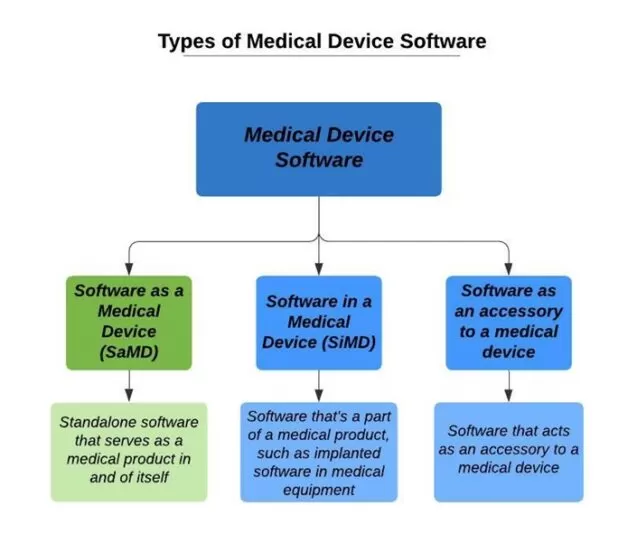
There are various types of software that are currently being used in healthcare. From EHRs to AI-powered chronic disease monitoring systems, medical device software can be classified as follows:
Source: https://www.johner-institute.com/articles/software-iec-62304/software-as-medical-device/
SaMD – standalone software that is a medical product itself and can be run with non-medical devices (e.g. laptops, smartphones, smartwatches, tablets, desktops, etc.)
Examples:
- Fertility apps: Natural Cycles
- Apps that can calculate medicine dosage depending on the patient’s current condition (e.g. diabetes control – eGlycemic Management System®)
- Apps for image viewing (e.g. MRI, X-Ray, etc.): CardioFlux, AIR™ Recon DL
SiMD – as its very name suggests, we are talking about software IN (=as part of) an existing medical device
Examples:
- Software that runs MRI or X-ray machines
- A mobile app that reads continuous data from a wearable (e.g. EKG monitor, blood glucose monitor, etc.)
General-purpose software that, by itself, is not a medical product.
Example:
- A mobile app for patient-clinician communication
- Software that encrypts data that is transmitted from a medical device
Software as accessories to medical devices
Example:
- Software used for the maintenance of a hardware medical device
SaMD: definition and differences between software types
In December 2013, IMDRF (International Medical Device Regulators Forum) defined SaMD as follows: “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware.” Put simply, SaMD is a medical device on its own.
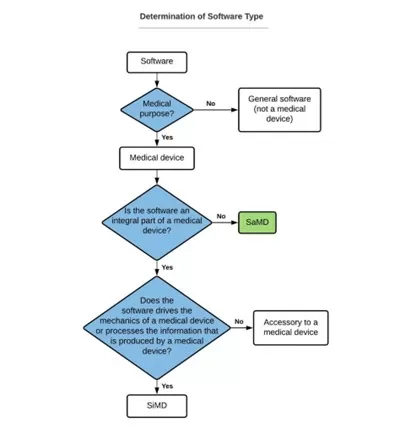
In order to determine the type of specific software, there are several questions that need to be answered:
Source: https://yan-chia.com/2021/10/15/medical-device-software-vs-software-as-a-medical-device-samd/
How does SaMD work?
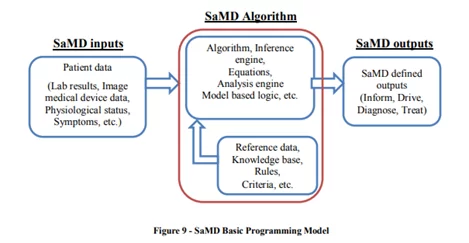
In 2017, IMDRF defined the context of the clinical evaluation process that involves SaMD. Basically, a SaMD uses an algorithm that operates on digital data, producing an output for the medical purpose defined by its manufacturer:
For example, CAD (computer-aided detection) software – such as cmAssist® – uses an algorithm that allows it to detect breast cancer or tumors by post-processing and analyzing images from multiple imaging modalities (e.g. mammography, MRI, CTs, ultrasonography, etc.) CAD systems use pattern recognition software that allows them to distinguish unfamiliar forms such as breast masses, breast density, or mass segmentation.
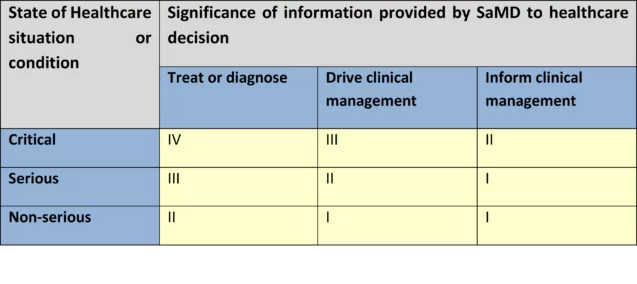
SaMD categorization
Based on the impact of the provided information on patient/public health, IMDRF establishes four SaMD risk categories:
As far as the Software Lifecycle Processes are concerned, the IEC 62304 standard establishes three additional safety classes:
- Class A: No injury or damage to health is possible
- Class B: Non-serious injury is possible
- Class C: Death or serious injury is possible
Additionally, since SaMD collects personal data, it also needs to be GDPR and HIPAA compliant.
SaMD: market size and perspectives
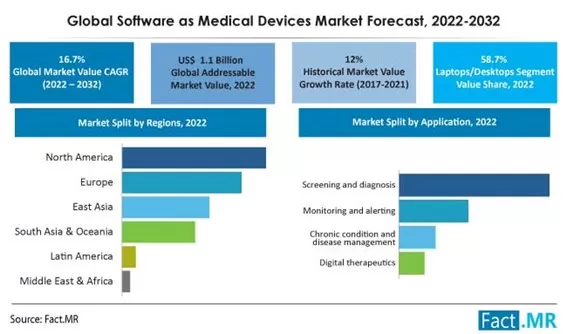
According to Fact.MR, between 2017 and 2021, the global market for SaMD registered a CAGR of 12% and will continue to grow at a CAGR of 16.7% between 2022 and 2032, reaching a valuation of $5.4 billion:
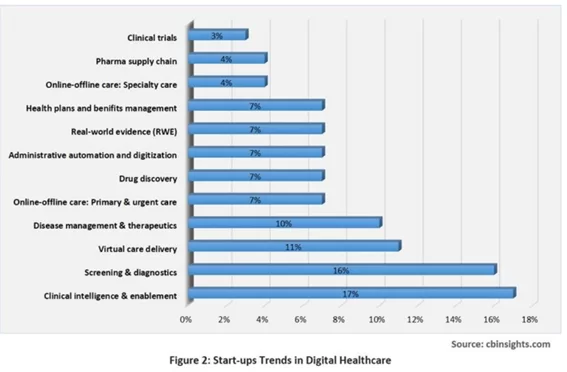
As highlighted by TATA ELXSI (based on a CB Insights report), SaMD is already playing a crucial role in prevalent areas such as clinical trials or online and offline specialty care:
The difference between Software as a Medical Device and Medical Device Software
As we have already seen, Medical Device Software has various subclasses out of which we can highlight three:
Source: https://yan-chia.com/2021/10/15/medical-device-software-vs-software-as-a-medical-device-samd/
To make it simple, SaMD can be defined as a subclass of Medical Device Software.
Medical Device Software and Digital Therapeutics
Under the Digital Health Solutions umbrella, digital therapeutics – or DTx – can be defined as a regulated sub-class of SaMD.
Source: https://capgemini-engineering.com/nl/en/integrated_solution/software-as-a-medical-device-samd-dtx/
DTx uses evidence-based and clinically-evaluated software to deliver therapeutic interventions directly to patients. DTx is used to prevent, manage, and treat a wide variety of diseases such as Alzheimer, type II diabetes, anxiety, asthma, ADHD, etc. Unlike fitness or wellness apps that are not usually regulated, don’t undergo scientific testing, are not based on clinical evidence, and can be downloaded freely, DTx must undergo rigorous testing, comply with the same standards and regulations as traditional medical treatments, can be prescribed by healthcare professionals either as standalone treatments or in conjunction with other drugs or therapies, and are reimbursable. As highlighted by EDPS, DTx allows patients to self-manage symptoms and improve clinical outcomes and quality of life. and other clinical endpoints. DTx uses digital devices such as apps, sensors, IoT, or VR to trigger behavioral changes.
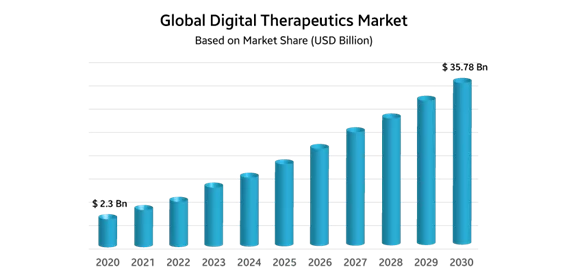
According to Strategic Market Research, by 2030, the global DTx market is expected to reach $35.78 billion, growing at a CAGR of 31.4%:
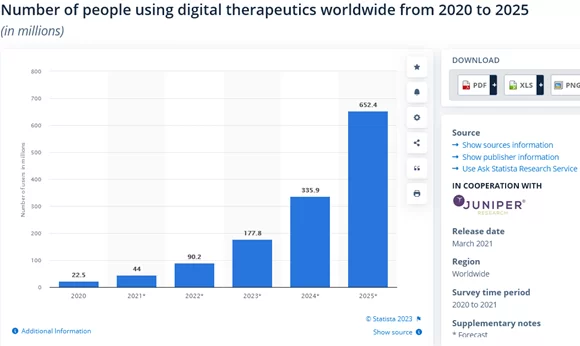
According to Statista, by 2025, over 652 million people are expected to use digital therapeutics:
SaMD: What you need to know in 2023
We have just entered 2023 and the future of SaMD continues to look bright. In order to be able to deliver consistent value, healthcare needs to keep embracing new technologies, interoperability possibilities, software innovation, etc. Statistics demonstrate that SaMD is one of the fastest growing sectors in digital healthcare, with key players such as Siemens Healthineers (AI-powered diagnostic, molecular medicine, therapeutic imaging, etc.), Medtronic (telehealth, diabetes treatment, AI-powered Remote Patient Monitoring solutions, etc.), or MindMaze (digital therapy for brain repair) investing heavily in SaMD development. These are only some examples of how SaMD is already rewriting the rules in healthcare delivery.
EU (Germany, Switzerland, Austria) and US Regulatory Considerations
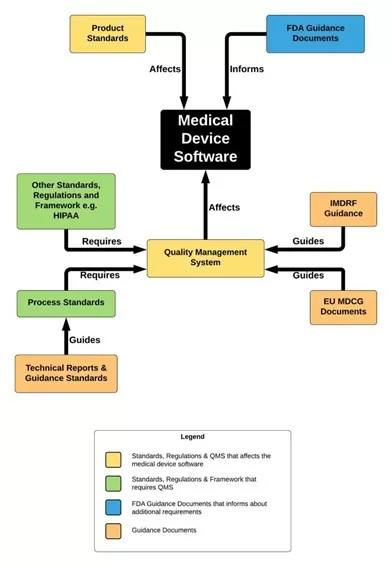
Given the prevalence of SaMD in the healthcare ecosystem, working on a common regulatory framework has become a pressing need, giving birth to the International Medical Device Regulators Forum (IMDRF). The guidance documents issued by IMDRF served as a base for the regulatory frameworks established by the E.U. and FDA. In her blog, Yan Chia offers a representation of the relationship between relevant standards, regulations, technical reports, and guidance documents:
For a more detailed relationship mapping, you can check out this document: https://yanchiacom.files.wordpress.com/2021/11/standards-regulations-and-guidance-documents.pdf
In December 2019, Germany adopted the Digital Healthcare Act (DVG), allowing healthcare professionals to prescribe Digital Health Applications (DiGA) that get approved by BfArM (through the “Fast Track” procedure) to all insured patients. DiGAs are Class I or IIa digital medical products that have a medical function.
Similar to Germany and in order to align the Swiss legislation regarding medical devices to the EU Medical Device Regulations (IVDR and MDR), Switzerland revised the Medical Devices Ordinance (MedDO) and established a new ordinance, CTO-MedD (Clinical Trials with Medical Devices.)
Since Austria is part of the European Union, the regulations for medical devices align with the European regulatory framework. BASG (Austrian Federal Office for Safety in Health Care) oversees that these regulations are met.
Wrap up
From the invention of the stethoscope in 1816 to the invention of the electrocardiograph, cardiac defibrillator, noninvasive fetal heart monitoring, MRI, or CT scanners, technological advancements have continuously supported medicine advancements, allowing it to adapt to new realities and demands. With healthcare undergoing an impressive digital transformation, the possibilities for SaMD are virtually endless, marking a watershed in ML analytics, 3D shape reconstruction, screening, disease diagnosing, monitoring, treatment, and prevention.