Artificial Intelligence (AI) is on the unprecedented rise in multiple domains at the moment. GlobalData projects the AI market to be valued at $97 billion by this year with an expected CAGR of over 19%. AI takes a significant part in the change of how modern medical devices look and operate. Among the most common use cases of AI in the medical device industry are data management (including predictive analytics and diagnostics assistance), remote surgery, clinical trials, and others.
AI technology in the medical device industry leverages the capability of computers to process vast quantities of data and learn from it, resulting in improved efficiency and the elimination of potential human mistakes through automation. New technologies require new standards and regulations, and medical device software standards have been subject to constant change in these. With AI it is often not possible to design documents due to the lack of guidance, standards, and even terminologies sometimes.
That is one of the challenges in AI adoption for medical devices. There are plenty. In this article, we’re going to explore common mistakes that software development companies should avoid when developing and implementing AI in medical devices.
We at Elinext have profound experience in developing software for healthcare devices and are always on a search for perfection in delivering the best products to our customers. That’s why it’s vital for us to explore miscues in AI adoption for medical devices.
Challenges and Mistakes in AI Adoption for Medical Devices
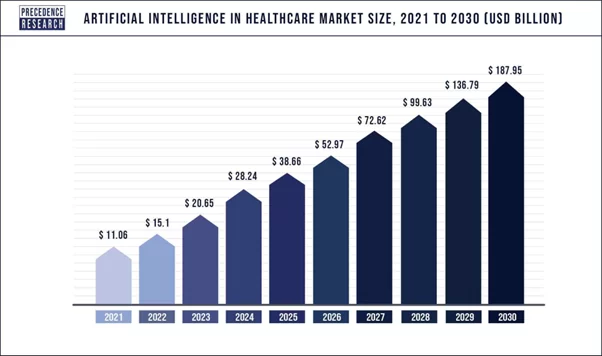
AI in the healthcare market size worldwide was capped at USD 15.1 billion in 2022, and it is expected to reach the mark of USD 187.95 billion by 2030.
AI in healthcare
At the same time, medical devices are greatly improving patient care and diagnostic accuracy, enhance patient outcomes, and streamline healthcare workflows. It’s only logical that integrating AI into medical devices is imminent. However, it can’t go without its challenges.
Challenge #1:
Lack of Regulatory Compliance
One of the most critical aspects of developing medical devices using AI is ensuring regulatory compliance. We briefly mentioned it in the introductory part of a blog post.
The healthcare industry is arguably one of the most regulated domains. Therefore medical device manufacturers have to follow a prolonged set of guidelines and regulations. In the US they’re controlled by the Food and Drug Administration (FDA).
The UK government via the Alan Turing Institute issued the act on the “responsible design and implementation of AI systems.”
France has established the French National Consultative Ethics Committee for Health and Life Sciences (CCNE) to provide ethical guidance in healthcare. They have published opinions and recommendations regarding the use of AI in healthcare, highlighting the importance of transparency, informed consent, and human oversight in AI systems.
Bundesministerium für Gesundheit, BMG is responsible for overall healthcare policy in Germany.
The point is, new regulatory documents appear with regularity, and being aware of them is a must for every medical device producer on the market.
Neglecting regulatory requirements can lead to costly delays in the development and adoption of devices, and consequently to rejections. On extreme occasions, they even lead to legal consequences.
That’s why we put lack of regulatory compliance as a #1 challenge for medical device manufacturers.
How to overcome:
To overcome the challenge of lack of regulatory compliance, it is essential for companies to have a designated responsible person or team who is knowledgeable about regulations and takes ownership of ensuring compliance. This person can stay updated on regulatory changes, interpret the requirements, and ensure that the company’s practices align with the applicable regulations.
Challenge #2:
Insufficient Data Quality and Quantity
AI algorithms by default need huge amounts of data for training and validation. Therefore, medical devices have to get as detailed and unskewed data as possible so that software development companies have access to the most. If the devices feed AI algorithms with “bad” data, it causes security and privacy issues, errors, inefficiencies with electronic health records, and other potential long-term damaging consequences. We’re not just talking about IoT and data gathered by medical devices. All types of medical data have to accurately represent the target patient population, ensuring that the AI algorithms perform optimally across different demographics. Neglecting data quality and quantity can lead to biased or inaccurate algorithms, which may compromise patient safety and effectiveness.
How to overcome:
Invest in data management technologies and resources, use preventive safeguards in all data systems, and offer data-related activities to healthcare workers. Researching AI technology and resources helps to bring about the desired results. Healthcare providers invest more in medical devices and use solutions that use human biometrics to address data issues. Consultants and tech specialists who ensure data quality in healthcare should keep a close eye on how data is gathered, stored, and used. System safeguards help prevent data entry errors and sustain a lot of data integrity. If the system flags duplicating data, that helps with the overall data verification. As staff members complete periodic training about data quality, they start to realize more about how mistakes could ultimately reduce the reliability of the available data. So training is a must.
Challenge #3:
Lack of Transparency and Understanding
The “black box” nature of AI algorithms can tame their acceptance pace in the healthcare industry.
Medical professionals and regulatory bodies require transparency and explainability to trust AI-powered medical devices.
Failure to prioritize explainability and transparency can limit the adoption of AI-powered medical devices and raise ethical concerns.
How to overcome:
Software development companies should focus on developing AI models that provide clear explanations for their decisions and predictions. Model interpretability, feature importance analysis, and decision boundary visualization as viable acting techniques should and will enhance the explainability of AI models. Google Trends Popularity Index skyrocketed the term “Explainable AI” by 2023 for a reason. Let’s explore one of the techniques. Model interpretability aims to provide insights into the internal workings of an AI model.
It involves analyzing the model’s structure, parameters, and activation patterns to understand how it processes and transforms input data to produce output predictions.
By examining the inner mechanisms of the model, researchers and practitioners can gain a better understanding of the factors that influence its decision-making process.
Medical device manufacturers and software developers to maintain the work of these devices should dive deeper into understanding AI models both for themselves and for the end-users (medical professionals) for seamless operations.
Challenge #4:
Neglecting Data Privacy and Security
AI-powered medical devices tend to handle sensitive patient data, making data privacy and security paramount.
All these regulations we discussed in the first point of this section are there for a reason. Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR), is crucial. So is HIPAA in North America.
Software development companies must implement robust security measures to safeguard patient information from unauthorized access.
Neglecting data privacy and security can result in loss of patient trust, legal repercussions, and damage to the reputation of the medical device and the software development company.
How to overcome:
To mitigate these risks, software development companies should prioritize data privacy and security throughout the development process.
This involves implementing encryption and access controls, conducting regular security audits, training employees on data protection practices, and establishing incident response plans to handle potential breaches effectively.
Data privacy and security are paramount in the field of AI-powered medical devices. Software development companies must adhere to strict security measures, comply with data protection regulations, and prioritize the trust and privacy of patients.
Challenge #5:
Limited Interoperability and Integration
Neglecting interoperability can result in siloed data, inefficient workflows, and hindered adoption of AI-powered medical devices. Medical devices must seamlessly integrate with existing healthcare systems such as electronic health records (EHRs), and other pieces of software. When medical devices cannot share data with other systems, the information they collect remains isolated within their own environment.
This leads to siloed data, where valuable patient information is gathered across different devices and software platforms. Siloed data hampers comprehensive patient care as healthcare providers may not have a complete view of a patient’s medical history and current condition. As for inefficient workflows, when medical devices do not seamlessly integrate with existing healthcare systems, healthcare professionals often face the challenge of manually transferring data between different systems.
This manual process is time-consuming and prone to errors, which can impact patient care and increase administrative burdens on healthcare providers. Inefficient workflows can also impede the timely delivery of care and delay decision-making processes.
How to overcome:
Standardization, APIs, harmonization of data, and continuous monitoring and improvement are the keys to great interoperability and integration.
Establishing Industry-wide standards for data formats, communication protocols, and interfaces is crucial to promote interoperability.
International organizations, such as HL7 and DICOM play a significant role in developing and maintaining standards for healthcare data exchange. AI-powered medical devices should adhere to these standards to ensure compatibility and interoperability with other systems.
Implementing well-defined APIs allows different devices and systems to communicate and exchange data.
Ensuring data harmonization across different medical devices is essential. This involves mapping and standardizing data elements, such as patient demographics, clinical measurements, and diagnostic codes, to enable seamless integration and interpretation of data from different sources.
Interoperability challenges may arise as technology evolves, new standards emerge, or healthcare workflows change. AI is potentially a game-changer, so continuous monitoring and improvement efforts are essential.
Conclusion
The adoption of AI in medical devices holds immense promise for revolutionizing healthcare. Software development companies like ours have a vital role in driving successful AI integration.
By avoiding common mistakes, such as neglecting regulatory compliance, overlooking data quality and privacy, lacking transparency, and limited interoperability, the development and implementation of AI-powered medical devices can and will make a positive impact on patient care. By addressing the aforementioned challenges head-on, software development companies can unlock the full potential of AI in healthcare and contribute to the advancement of medical technology. The path to successful AI adoption in medical devices is paved with attention to detail, collaboration with domain experts, and a commitment to patient safety and care.